Where is the heat?
The combustion process is no mystery. Mix fuel and oxygen, provide a spark and you get a bunch of heat and volume expansion. The internal combustion engine (and you could argue all of the industrialized world) relies on that volume expansion to produce mechanical work. The volume expansion is the payload, it is the whole point of the engine. The heat on the other hand...that is more of a nuisance. In fact, engineers go to great pains to dump the heat as quickly as possible and minimize its impact on engine performance.
The ultimate source of heat is the breaking of molecular bonds in fuel and oxygen molecules that we see as burning gas in the cylinder chamber. That hot gas is in contact with the cylinder wall and transfers heat (about half the total heat produced) into the metal of the block and piston. The other half stays in the gas and exits the engine as exhaust gas.
Heat Recovery in Engine Coolant
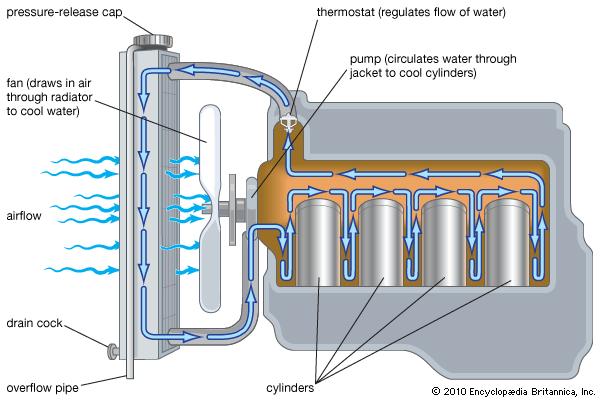
Diagram of engine coolant loop
Without any intervention, excessive heat can build up in the engine block and cause major problems. Seals wear out faster, lubricants breakdown, even metal can weaken and fail. To keep the heat from building up in the engine block, engineers carefully design fluid loops that carry heat away from the engine. That brings us to the first source of heat in an engine: the engine coolant.
Coolant is circulated through the engine block and cooled by a radiator, usually located in the front of the vehicle. Roughly half of the wasted heat in an engine (so about 30% of the total input energy) is transferred through the engine block and into the coolant.
Generally, the coolant leaves the engine block at around 100C, which is fairly low as far as typical heat recovery options are concerned. This low temperature limits the options for what we can do with the heat. On the plus side, the coolant is already conveniently in liquid form (See our page on Heat Recovery for more info) and circulates to a central point (the radiator). This opens up possibilities like an aftermarket heat recovery radiator that can drop in and replace a traditional radiator.
Heat Recovery in Exhaust
The gas coming out of a car’s tailpipe is a combination of carbon dioxide, water vapor, nitrogen, and a host of other molecular byproducts from the combustion process. As anyone who has burned themselves on an exhaust pipe can tell you, exhaust is hot. In fact the exhaust is typically around 650C [LINK].
Diagram of a typical exhaust system
This temperature is great, but being in the gaseous state means that the heat density is relatively low, requiring more surface area to extract the heat (Again, see Heat Recovery for more info). That means more equipment under the car and if you have ever taken a gander at the underside of a car you know that space is at a premium.
As the diagram shows, an exhaust system entails more than just a pipe connected to your engine. There is a catalytic converter, to reduce the harmful NOx, as well as a muffler and resonator to reduce the noise of the gas.
You could imagine lining the exhaust pipe with a material to capture heat or replacing it altogether with an specialized heat recovery pipe system. Or you could replace a traditional component like the muffler, with a device that both muffles and recovers heat. One word of caution though, any obstructions put into the exhaust stream can make it more difficult for exhaust gas to escape, impacting the operation and/or life of the engine. The last thing you want to do is improve your engine efficiency at the cost of reliability.
What can heat do?
At this point, we have established that there is a bunch of heat available in an automobile engine. Great. Now what can we do with it?
Unlike a stationary heat recovery application, a car is a closed system, so we can’t export excess heat or electricity to some other process. We have to use it all right then and there. Fortunately, there are two key systems in an automobile that can benefit from additional power.
Add horsepower
More horsepower is always better than less right? In this case we aren’t just talking about making a car get better pickup. By adding horsepower, a heat recovery system makes an engine more efficient. This means that for the same performance (HP output to the wheels) a heat recovered engine could operate at a lower load, reducing the fuel usage and emissions. If heat recovery is factored in from the design phase, engineers could even specify a smaller engine, knowing that heat recovery will provide additional horsepower.
Conveniently, the demand for horsepower generally aligns with the availability of heat. As a car accelerates, the engine is outputs power, generating more heat, which can be converted into horsepower and sent back to the wheels.
Power electronic accessories
To power the accessories (radio, lights, windows etc) a car needs electricity. Most cars have an alternator, a small generator that charges your battery while driving. Once the engine is started, the alternator pulls some mechanical power from the engine through a belt and converts it to electrical power. If there were another way to produce electricity to power the accessories, we could essentially disconnect the alternator and give that horsepower back to the engine.
The battery offers a great buffer between the heat availability and electricity demand. A heat recovery system can feed into the battery when the heat is available then the electricity can be used by the accessories whenever it is needed.
In conclusion
Now you can start to see a gap. On one side we have two heat sources, coolant and exhaust. On the other, we have two uses of energy in a car, horsepower and electricity. The question now is, how do we connect the heat sources with the energy use? That is where technology comes in and where we will pick up in the third and final part of this series.
Matt Gutschow
Co-Founder, HeatCalc